Abstract
Introduction Despite recent therapeutic advances Multiple Myeloma (MM) remains largely incurable, and outcomes in patients who develop resistance to imid or proteasome inhibitor therapies are universally dismal.1 Allogeneic hematopoietic cell transplant (alloHCT) remains the only curative MM treatment but has been associated with historically high rates of GVHD and of non-relapse mortality (NRM), exceeding 40% in some series.2 Although these rates have decreased in recent years, the potential morbidity and mortality associated with alloHCT and the increasing availability of alternative non-transplant therapies demands a thoroughly informed pre-alloHCT assessment. Here we assess the impact of pre-alloHCT variables on clinical outcomes in a large cohort of relapsed/refractory (RR) MM patients who underwent CD34+ selected alloHCT at our institution.
Methods This retrospective study included all MM patients who had CD34+ selected alloHCT from Jun 2010 to Dec 2015. Patients were conditioned with targeted dose busulfan (0.8 mg/kg x 10), melphalan (70 mg/m2 x 2) and fludarabine (25mg/m2 x 5) followed by infusion of a CD34+ selected peripheral blood stem cell graft, without post alloHCT GVHD prophylaxis. Estimates were given using the Kaplan-Meier and cumulative incidence methods. Competing risks for relapse, NRM, and GVHD were death, relapse, and relapse or death respectively. The log-rank and Gray's test were used to assess univariable associations. GVHD by 6 months was assessed via a landmark analysis.
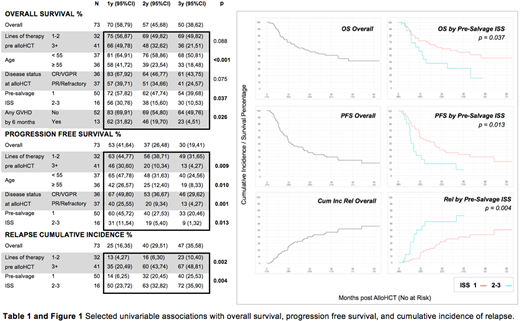
Results Our 73 patient cohort had a median age of 55 (37-66) and was mostly male (74%). Most patients had low risk MM by ISS (50/66, 76%) and intermediate risk MM by R-ISS (45/66, 68%) at pre-salvage assessment. Patients had a median of 4 (2-9) pre-alloHCT lines of therapy and were evenly split between patients in PR and in VGPR or CR at time of alloHCT (50% and 49%). Median HCT-CI score was 2 (range 0-6) with the majority of patients graded as intermediate or high risk (score ≥1; 55/73, 75%).
At a median follow-up in survivors of 35 months (12-84) OS and PFS rates were 70% and 53% at 1 year (95% CI 58-79, 41-64) and 50% and 30% at 3 years, respectively (38-62, 19-41). The cumulative incidences of relapse were 25% and 47% at 1 and 3 years, respectively (16-35, 35-58), and 1 year NRM was 22% (13-32). Deaths were balanced between relapse and non-relapse causes (54% and 46% respectively). Incidence of grade II-IV acute GVHD was 7% at 100 days (3-14), and of chronic GVHD was 8% at 1 year (3-16).
In univariable analysis, intermediate-high risk ISS assessed prior pre-alloHCT salvage therapy was associated with lower OS (3 year 30 v 54%, p=0.037), lower PFS (3 year 9 v 33%, p=0.013), and greater relapse incidence (3 year 72 v 39%, p=0.004). Older age and GVHD prior to 6 months were also associated with lower OS; older age, more heavily pre-treated disease, and worse disease status at alloHCT were associated with lower PFS; and heavier pre-alloHCT treatment was also associated with higher relapse (Table 1). Higher HCTCI was not associated with increased NRM (1 year 22 v 16 v 27% for HCTCT 0, 1-2, ≥3 respectively; p = 0.863).
Discussion We describe a cohort of high-risk heavily pretreated RRMM patients with durable OS (50% at 3 years), comparatively low PFS (30% at 3 years), and historically improved rates of NRM (22% at 1 year). We also importantly identified numerous pre-alloHCT variables that were associated with survival, PFS, and relapse. Amongst these, poor ISS measured prior to pre-alloHCT salvage was consistently associated with worse survival and relapse outcomes and may speak to this score's utility as a dynamic measure of disease risk in patients exposed to multiple lines and therapy.
Conclusions Our report reinforces that CD34+ selected alloHCT can achieve prolonged disease control and long term survival in high risk, heavily treated refractory MM populations, and newly describes certain pre-transplant variables that may help identify patients with better potential survival and relapse outcomes. Given the dismal prognosis and lack of established alternate therapies for RRMM patients, we advocate that identification of favorable or adverse pre-transplant variables during pre-alloHCT assessment be used to inform alloHCT decision-making rather than to exclude certain patient cohorts from this potentially effective and curative treatment option.
Perales:Abbvie: Other: Personal fees; Merck: Other: Personal fees; Incyte: Membership on an entity's Board of Directors or advisory committees, Other: Personal fees and Clinical trial support; Takeda: Other: Personal fees; Novartis: Other: Personal fees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal